A 30-year-old woman rolls into your resuscitation bay looking very dyspneic on a non-rebreather, clammy with a heart rate of 135 bpm. She takes oral contraceptives, has had a sudden syncopal episode, and now lies in the stretcher struggling. Her blood pressure is 100/60 and she is hypothermic with a temp of 35.7°C. Her ECG and PoCUS suggest right heart strain. CTPA confirms a saddle pulmonary embolism (PE). But she’s not hypotensive… yet. So, what’s next? How do you predict which intermediate-risk patients will suddenly deteriorate? What role do biomarkers, imaging, and hemodynamics play in decision-making? Should she receive anticoagulation alone, or is thrombolysis warranted? When should you consider catheter-directed or surgical interventions? This case focuses us to think critically about risk stratification and early interventions in PE. Not all patients fit neatly into classification boxes, making clinical judgment crucial. Join Dr. Lauren Westafer, Dr. Justin Morgenstern, Dr. Bourke Tillman and Anton as they explore the key decision points, pitfalls, and lifesaving strategies for managing intermediate-risk PE in the ED…
Podcast production, sound design & editing by Anton Helman; Voice editing by Braedon Paul
Written Summary and blog post by Sara Brade, edited by Anton Helman April, 2025
Cite this podcast as: Helman, A. Morgenstern, J. Tillmann, B. Westafer, L. Intermediate Risk Pulmonary Embolism Risk Stratification, Management and Algorithm. Emergency Medicine Cases. Month, 2024. https://stg-emergencymedicinecases-emcstaging.kinsta.cloud/intermediate-risk-pulmonary-embolism-risk-stratification-management. Accessed July 2, 2025
Pulmonary embolism risk categories
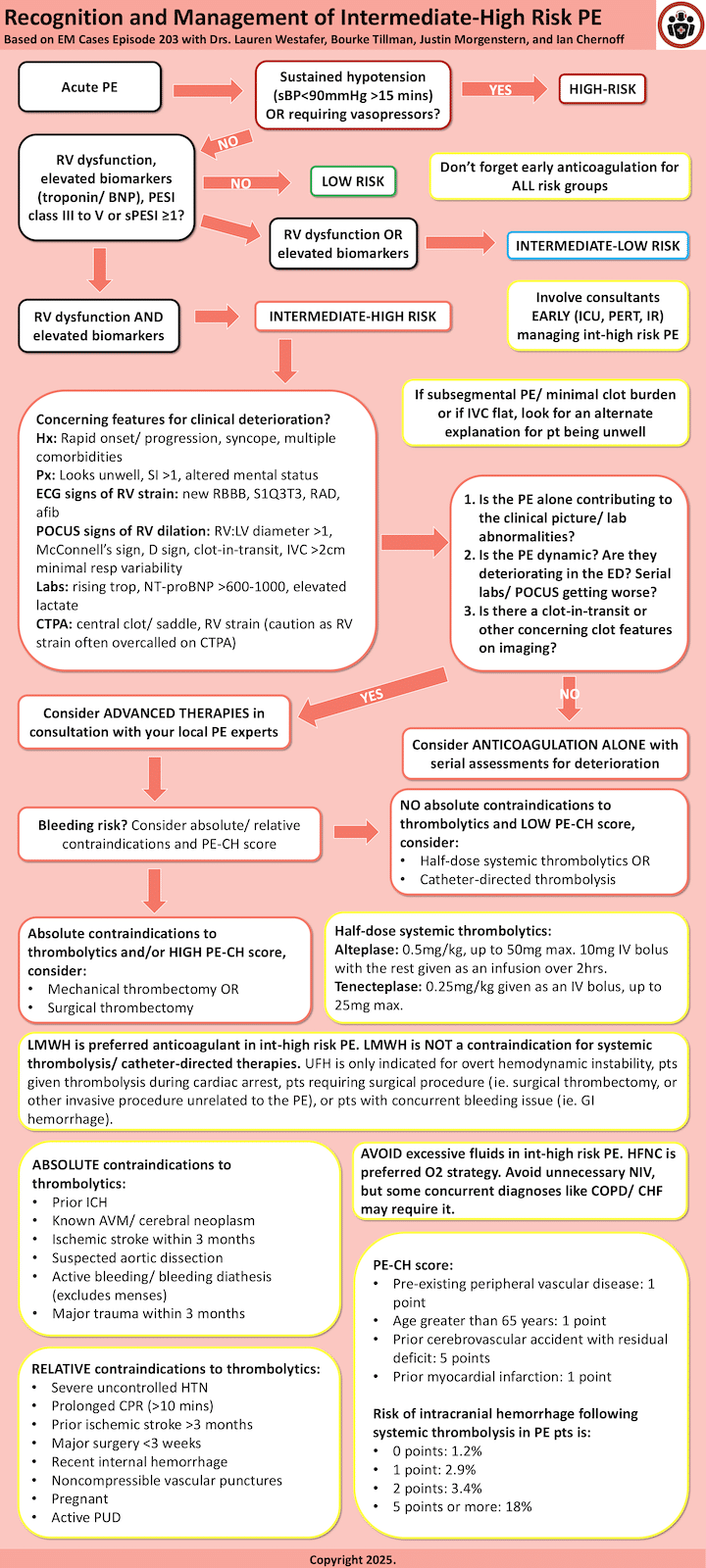
PE severity exists on a spectrum, ranging from low-risk cases to cardiac arrest. Patients who fall in the intermediate-risk category are particularly challenging because they represent a heterogenous group with varying degrees of severity and risk for clinical deterioration.
The European Society of Cardiology (ESC) classifies PE severity into four categories:
- Low-risk patients do not require oxygen, show no signs of RV dysfunction, and have normal biomarkers.
- Intermediate-low risk patients have either elevated biomarkers OR RV dysfunction but not both.
- Intermediate-high risk patients exhibit both elevated biomarkers AND RV dysfunction.
- High-risk patients have prolonged hypotension (systolic BP <90 mmHg for at least 15 minutes), require pressor support, or cardiac arrest.
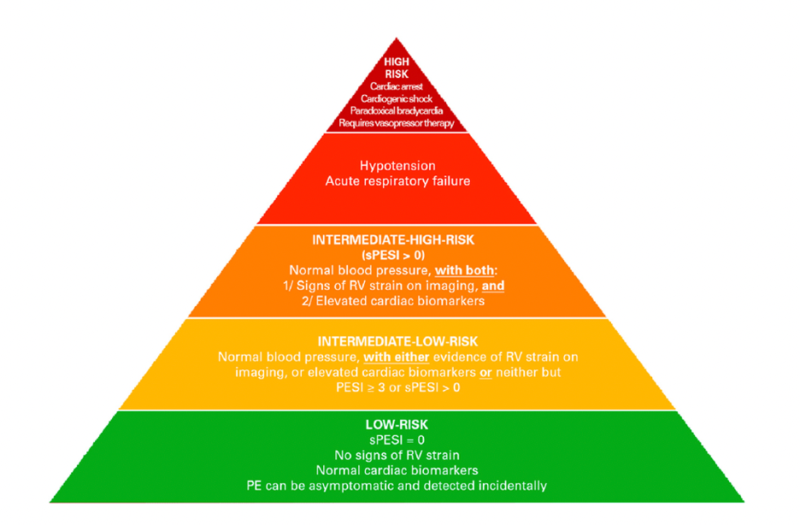
Source: https://doi.org/10.1161/CIRCINTERVENTIONS.116.00434
Mortality for intermediate-risk PE patients has been reported as high as 15% within the first 30 days. The challenge in the ED is identifying and treating those at the highest risk of deterioration before they progress to hemodynamic instability.
The pulmonary embolism death spiral: understanding how patients decompensates helps risk stratify them
In cases of clinically significant high risk and intermediate high risk pulmonary embolism, the clot is thought to increase pulmonary vascular resistance, forcing the right ventricle (RV) to work harder to pump blood forward. Since the RV is not structurally designed to handle increased afterload, it begins to dilate. This dilation leads to a vicious cycle where the RV’s myocardial perfusion is compromised, further reducing its contractility.
As the obstruction worsens, blood return to the left ventricle (LV) is diminished, reducing cardiac output. The dilated RV also physically compresses the LV, worsening cardiac output even further. Additionally, hypoxia from pulmonary vasoconstriction exacerbates myocardial ischemia. If this process continues unchecked, it can culminate in obstructive shock and cardiac arrest.
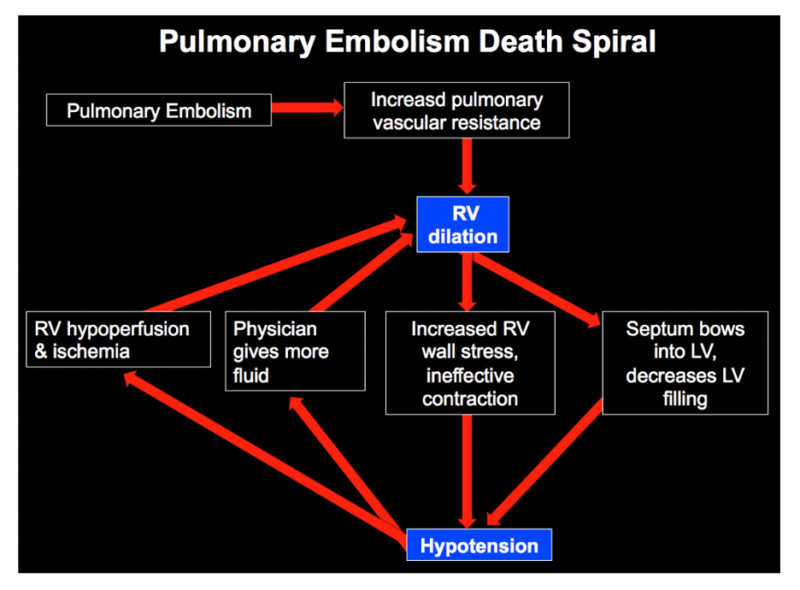
Source: Internet Book of Critical Care
Risk Stratification for acute decompensation in intermediate-risk PE
Assessing risk in intermediate-risk patients is complex and requires consideration of multiple factors, including history, exam findings, ECG, POCUS, biomarkers, and imaging. While risk scores like PESI, sPESI, BOVA, and Hestia are useful for predicting 30-day mortality, they are not designed to predict acute decompensation in the ED. Instead, clinicians should assess for a multitude of high risk features outlined below to inform their decision making with regards to thrombolysis, interventional procedures and escalation to ICU care.
High risk clinical features from history and physical for acute decompensation in pulmonary embolism
- Rapid symptom onset/progression
- Syncope: associated with RV dysfunction, early mortality, and increased risk of PE-related adverse events. While it is not necessarily an independent predictor of decompensation, its presence warrants caution.
- Comorbidities: Comorbidities also play a role in prognosis. The PESI score, which includes cancer, congestive heart failure, and chronic lung disease, predicts 30-day mortality but is not specifically designed for acute deterioration in the ED.
- Physical Exam:
- Clammy, cool extremities (suggesting obstructive shock), altered mental status
- Shock index >1 (HR/sBP) → higher short-term mortality risk
- Relative hypotension in patients with a history of hypertension
- O2 sat <90%, RR ≥30 bpm
ECG finding predictors of poor outcomes in pulmonary embolism
While these ECG features are not predictive of the diagnosis of PE, they do suggest RV dysfunction. The following findings, when new, are predictive of acute decompensation/prognosis:
- S1Q3T3
- Right axis deviation/RBBB
- Atrial fibrillation
PoCUS findings suggestive of RV dysfunction to predict acute decompensation in pulmonary embolism
Bedside ultrasound provides rapid assessment of RV function, and can provide valuable information to assess for other causes of shortness of breath. Key findings include:
- RV dilation (RV:LV ratio >1), best seen in the apical 4-chamber view.
- McConnell’s sign, characterized by RV free wall hypokinesis with apical sparing.
- D-sign, where the interventricular septum flattens due to increased RV pressure.
- Plethoric IVC, suggesting elevated right atrial pressures.
- Clot-in-transit, which carries a high mortality risk of 45%
Predictive value of biomarkers for acute decompensation in pulmonary embolism
Troponin elevation as a predictor of poor outcomes in pulmonary embolism
There is no clear cut-off for high-sensitivity troponin for risk stratification in patients with pulmonary embolism. Troponin can be elevated in the setting of PE for non-PE related reasons like AKI, CKD, and heart failure. While elevated troponin does correlate with RV dysfunction and may portend poor short-term morbidity/mortality outcomes, it remains unclear whether elevated troponin is an independent risk factor for these outcomes and what is the optimal cut-off value. Further, while a negative troponin in normotensive patients may be associated with more favourable short-term outcomes, negative troponin does not rule out significant RV strain/significant PE that may be at risk for acute decompensation.
BNP elevation as a predictor of poor outcomes in pulmonary embolism
Elevated BNP may confer an increased risk of short-term morbidity/mortality and correlates with severity of RV dysfunction; however, BNP can be elevated for non-PE reasons like baseline heart failure. If BNP is elevated, it is important to establish if PE is the cause for elevated BNP and review the clinical history and look for prior echocardiography reports/ prior BNP levels. In normotensive patients with PE, elevated BNP is poorly specific for predicting poor early short-term outcomes; however, in these same patients a normal BNP is highly sensitive for excluding poor early short-term outcomes. NT-proBNP <500pg/mL has been suggested as a low-risk cut off value. NT-proBNP >600-1000pg/mL has been suggested as a high-risk cut off value.
D-dimer elevation as a predictor of poor outcomes in pulmonary embolism
There is some evidence elevated D-dimer is associated with increased in-hospital mortality, but unclear how this relates to acute decompensation. Our experts do not rely on height of D-dimer elevation to assess risk of acute decompensation.
Lactate elevation as a predictor of poor outcomes in pulmonary embolism
Lactate ≥ 2mmol/L is associated with an increase in short-term mortality.
CTPA findings – can they predict acute outcomes in pulmonary embolism?
Clot burden has different implications in different patients. In otherwise low-risk patients, clot burden is not an independent predictor of poor outcomes. In high-risk patients, if clot burden is minimal, this should trigger a cognitive pause to search for alternate explanations for the patient’s clinical picture.
In intermediate-risk patients, high clot burden (ie. saddle PE, or central occlusion) may confer higher risk of poor short-term outcomes.
Pitfall: Beware that RV strain on CTPA is often overcalled, one study demonstrates a specificity of only 4% for RV strain compared to TTE.
Management of intermediate risk pulmonary embolism patients
Intermediate-low risk patients typically require anticoagulation alone, while intermediate-high risk patients may require thrombolysis, catheter-directed therapy, or thrombectomy, and ICU level care.
Key questions to guide management and assess high risk features outlined above
- Is the PE causing the clinical picture, or is there another underlying cause? e.g. do they have a subsegmental PE and some other disease causing them to be hypoxic/ tachy/ unwell (ie. COPD, HF, pneumonia) or do they have a saddle PE with a plethoric IVC and no other contributing comorbidities/ alternate diagnoses?
- Is the patient deteriorating/dynamic? Worsening BP, increasing HR, rising troponin, worsening RV findings on PoCS over time all suggest clinical progression.
- What is the patient’s bleeding risk? Use absolute contraindications to thrombolysis and the PE-CH score to assess the likelihood of intracranial hemorrhage (ICH).
- Is there a clot-in-transit? If so, thrombolysis or surgical embolectomy is indicated.
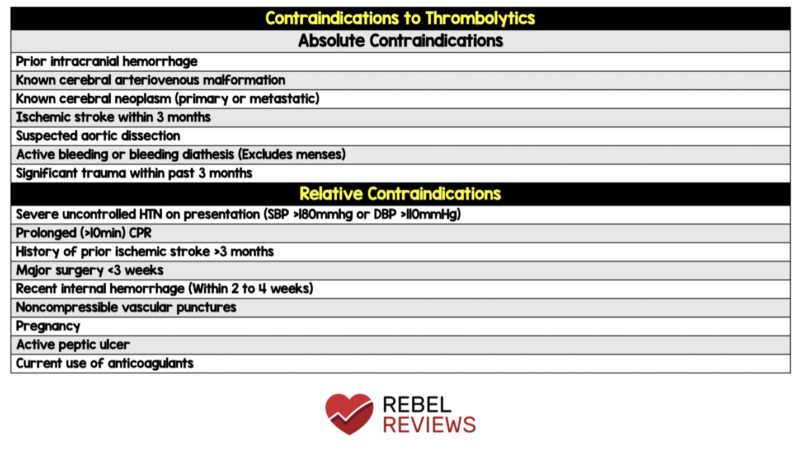
Source: https://rebelem.com/rebel-review/rebel-review-37-contraindications-to-thrombolytics/contraindications-to-thrombolytics/
PE-CH Score to assess risk of ICH in pulmonary embolism thrombolysis
Anticoagulation strategies in intermediate risk pulmonary embolism
For most intermediate-risk patients, low-molecular-weight heparin (LMWH) is preferred over unfractionated heparin (UFH), as studies suggest improved outcomes, fewer bleeding complications and it is less cumbersome to administer/less resource intensive. Unfractionated Heparin (UFH) may be preferred if:
- Cardiac arrest or persistent shock
- Invasive procedure (eg LP) or surgery (eg appendectomy) is required (this does not include mechanical embolectomy/catheter-based PE procedures)
- Active major bleeding
LMWH use is not a contraindication to thrombolysis!
In patients receiving thrombolysis, anticoagulation should be resumed or started after a minimum of 2 hours.
Oxygenation strategies in intermediate risk pulmonary embolism
An important general principle in the management of patients with pulmonary embolism is to avoid PEEP and/or endotracheal intubation whenever possible as RV dysfunction will predictably worsen.
High-flow nasal cannula (HFNC) is recommended for patients requiring >2-5L nasal prongs to maintain oxygen saturation.
Although non-invasive ventilation (NIV) may exacerbate RV dysfunction, it can be beneficial for patients with concurrent conditions like COPD or CHF.
Fluids in management of pulmonary embolism
Pitfall: Excess IV fluids is likely worsen RV dysfunction and should be avoided whenever possible.
Systemic thrombolysis or catheter-directed procedure for intermediate-risk pulmonary embolism?
Systemic thrombolysis or catheter-directed procedures should be considered in the intermediate high-risk patient after high risk feature assessment and answering the questions: is the pulmonary embolism accounting for the entire clinical picture? Is the patient dynamic? What is their bleeding risk? Is there a clot in transit?
Thrombolytics can be administered systemically or via catheter devices. Catheter-directed therapy delivers a smaller thrombolytic dose directly to the clot, potentially reducing intracranial bleeding risk. However, high-quality RCTs are lacking, and available studies are industry-funded, lack comparison groups, and focus on disease-oriented outcomes only.
For intermediate-high risk patients without contraindications to thrombolytics, the PERT Consortium recommends catheter-directed thrombolysis or reduced-dose systemic thrombolysis. If absolute contraindications exist, mechanical or surgical embolectomy should be considered, particularly for centrally located clots. Avoid thrombolytics in patients with a high PE-CH score.
The PEITHO trial found that thrombolysis with tenecteplase in intermediate high risk patients reduced hemodynamic decompensation (2.6% vs. 5.6%) but increased intracranial hemorrhage (2%) and extracranial bleeding. A meta-analysis found no clear mortality benefit of systemic thrombolytics in intermediate-risk PE.
For systemic thrombolysis, no strong evidence supports one drug (tenecteplase vs. alteplase), dose, or regimen over another. Drug selection typically depends on hospital availability. The PERT Consortium recommends reduced-dose thrombolysis to lower bleeding risk while maintaining efficacy, though studies on this approach were underpowered. Our experts suggest using half-dose or less when opting for systemic thrombolysis.
For patients deemed appropriate for thrombolysis, reduced-dose thrombolytics are preferred by our experts to minimize bleeding risk:
- Half-dose alteplase: 0.5 mg/kg (max 50 mg) as 10 mg bolus with remaining dose over 2 hours.
- Half-dose tenecteplase: 0.25 mg/kg (max 25 mg) as a single bolus.
Surgical embolectomy is recommended by the ESC for high-risk patients who fail thrombolysis or have contraindications, especially in cases of clot in transit, offering similar efficacy but lower bleeding risk.
Management of intermediate-high risk PE is complex, influenced by institutional protocols and resource availability. Early consultation with Pulmonary Embolism Response Team (PERT), ICU and/or interventional specialists is crucial!
Key take home points for management of intermediate risk pulmonary embolism
- PE exists on a spectrum of severity that is challenging to capture in discrete categories.
- Intermediate-high risk patients are defined by both elevated biomarkers and evidence of RV dysfunction.
- PE risk scores do not apply to intermediate risk PE patients for predicting acute decompensation.
- LMWH is the first line medication choice for patients with intermediate risk PE. UFH is only indicated for patients who received thrombolysis during cardiac arrest, those with persistent shock, those going for an invasive surgical procedure (not catheter directed thrombolysis or mechanical thrombectomy), or those with a concurrent major bleeding.
- Features that may raise your suspicion for clinical deterioration in the ED include: rapid onset/ progression of symptoms, syncope, shock index >1, high O2 requirements, relative hypotension, altered mental status, elevated troponin/ BNP/ lactate, signs of RV strain on ECG (afib, new RBBB/RAD, S1Q3T3), PoCUS findings of RV dilation, CTPA high clot burden / saddle PE.
- After assessing for high risk features, ask 4 questions and involve consultants early to guide decision for systemic thrombolysis or catheter-based procedures:
- Is the PE causing the clinical picture, or is there another underlying cause?
- Is the patient deteriorating/dynamic?
- What is the patient’s bleeding risk? Use absolute contraindications to thrombolysis and the PE-CH score
- Is there a clot-in-transit?
- There is no high-quality, industry-free evidence of benefit for catheter-based procedures in intermediate risk PE patients compared to systemic thrombolysis; these procedures should be reserved for the intermediate high risk and high risk pulmonary embolism patient with absolute contraindications to systemic thrombolysis or a high PE-CH score.
- Our experts recommend 1/2 dose systemic thrombolytics to minimize the risk of major bleeding complications compared to full dose thrombolytics.
- Avoid excessive fluid administration and PEEP/endotracheal intubation whenever possible. HFNC are indicated for patients failing NP.
In part 2 of this 2-part podcast series on Risk Stratification and Management of Pulmonary Embolism Drs Helman, Westafer, Morgenstern and Tillmann delve into the High Risk Pulmonary Embolism patient – a category that also includes a heterogenous group of patients and requires a nuanced approach.
First10EM evidence-based deep dive on intermediate and high risk PE by Dr. Justin Morgenstern
References
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). European Heart Journal. 2020;41(4):543-603. doi:10.1093/eurheartj/ehz405
- Barco S, Ende-Verhaar YM, Becattini C, et al. Differential impact of syncope on the prognosis of patients with acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2018;39(47):4186-4195. doi:10.1093/eurheartj/ehy631
- Ozsu S, Erbay M, Durmuş ZG, Ozlu T. Classification of high-risk with cardiac troponin and shock index in normotensive patients with pulmonary embolism. J Thromb Thrombolysis. 2017;43(2):179-183. doi:10.1007/s11239-016-1443-3
- Kabrhel C, Rosovsky R, Garvey S. Special Considerations in Pulmonary Embolism: Clot-in-Transit and Incidental Pulmonary Embolism. Crit Care Clin. 2020;36(3):531-546. doi:10.1016/j.ccc.2020.02.008
- Islam M, Nesheim D, Acquah S, et al. Right Heart Thrombi: Patient Outcomes by Treatment Modality and Predictors of Mortality: A Pooled Analysis. J Intensive Care Med. 2019;34(11-12):930-937. doi:10.1177/0885066618808193
- Agterof MJ, Schutgens REG, Snijder RJ, et al. Out of hospital treatment of acute pulmonary embolism in patients with a low NT-proBNP level. J Thromb Haemost. 2010;8(6):1235-1241. doi:10.1111/j.1538-7836.2010.03831.x
- Lankeit M, Jiménez D, Kostrubiec M, et al. Validation of N-terminal pro-brain natriuretic peptide cut-off values for risk stratification of pulmonary embolism. Eur Respir J. 2014;43(6):1669-1677. doi:10.1183/09031936.00211613
- Song ZK, Wu H, Xu X, et al. Association Between D-Dimer Level and In-Hospital Death of Pulmonary Embolism Patients. Dose Response. 2020;18(4):1559325820968430. doi:10.1177/1559325820968430
- Vanni S, Nazerian P, Bova C, et al. Comparison of clinical scores for identification of patients with pulmonary embolism at intermediate-high risk of adverse clinical outcome: the prognostic role of plasma lactate. Intern Emerg Med. 2017;12(5):657-665. doi:10.1007/s11739-016-1487-6
- Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142(6):1417-1424. doi:10.1378/chest.11-2739
- O’Hare C, Grace KA, Schaeffer WJ, et al. Adverse Clinical Outcomes Among Patients With Acute Low-risk Pulmonary Embolism and Concerning Computed Tomography Imaging Findings. JAMA Netw Open. 2023;6(5):e2311455. doi:10.1001/jamanetworkopen.2023.11455
- Lyhne MD, Giordano N, Dudzinski D, et al. Low concordance between CTPA and echocardiography in identification of right ventricular strain in PERT patients with acute pulmonary embolism. Emerg Radiol. 2023;30(3):325-331. doi:10.1007/s10140-023-02130-z.
- Westafer LM, Presti T, Shieh MS, et al. Trends in Initial Anticoagulation Among US Patients Hospitalized With Acute Pulmonary Embolism 2011-2020. Annals of emergency medicine 2024;84:518-29.
- Jimenez D, de Miguel-Diez J, Guijarro R, et al. Trends in the Management and Outcomes of Acute Pulmonary Embolism: Analysis From the RIETE Registry. Journal of the American College of Cardiology 2016;67:162-70.
- Prucnal CK, Jansson PS, Deadmon E, Rosovsky RP, Zheng H, Kabrhel C. Analysis of Partial Thromboplastin Times in Patients With Pulmonary Embolism During the First 48 Hours of Anticoagulation With Unfractionated Heparin. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine 2020;27:117-27.
- Silver MJ, Gibson CM, Giri J, et al. Outcomes in High-Risk Pulmonary Embolism Patients Undergoing FlowTriever Mechanical Thrombectomy or Other Contemporary Therapies: Results From the FLAME Study. Circ Cardiovasc Interv. 2023;16(10):e013406. doi:10.1161/CIRCINTERVENTIONS.123.013406
- Konstantinides SV, Vicaut E, Danays T, et al. Impact of Thrombolytic Therapy on the Long-Term Outcome of Intermediate-Risk Pulmonary Embolism. J Am Coll Cardiol. 2017;69(12):1536-1544. doi:10.1016/j.jacc.2016.12.039
- Mathew D, Seelam S, Bumrah K, Sherif A, Shrestha U. Systemic thrombolysis with newer thrombolytics vs anticoagulation in acute intermediate risk pulmonary embolism: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23(1):482. doi:10.1186/s12872-023-03528-w
- Rivera-Lebron B, McDaniel M, Ahrar K, et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037. doi:10.1177/1076029619853037
- Westafer LM, Presti T, Shieh MS, et al. Trends in Initial Anticoagulation Among US Patients Hospitalized With Acute Pulmonary Embolism 2011-2020. Annals of Emergency Medicine. 2024;84(5):518-529. doi:10.1016/j.annemergmed.2024.05.009
- Robertson L, Jones LE. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst Rev. 2017;2(2):CD001100. doi:10.1002/14651858.CD001100.pub4
- Messika J, Goutorbe P, Hajage D, Ricard JD. Severe pulmonary embolism managed with high-flow nasal cannula oxygen therapy. Eur J Emerg Med. 2017;24(3):230-232. doi:10.1097/MEJ.0000000000000420
Drs. Helman, Westafer, Morgenstern & Tillmann have no conflicts of interest to declare
Now test your knowledge with a quiz.




Leave A Comment